News
New guidance on transcranial magnetic stimulation echoes Council’s call for more evidence collection
New guidance from The National Institute for Health and Care Excellence (NICE) on the use of transcranial magnetic stimulation (TMS) reflects some key recommendations of the Council’s report on novel neurotechnologies.
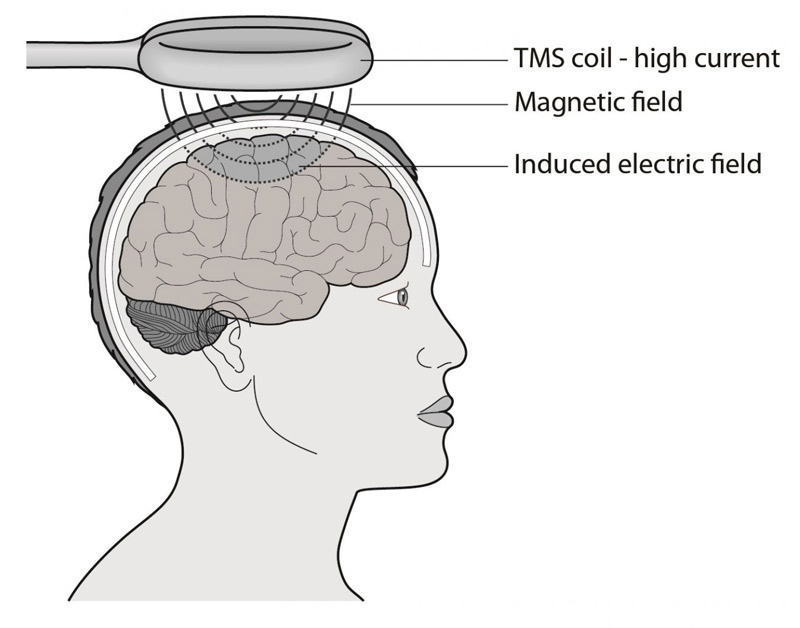
Find out more about how TMS works
The guidance, issued to the NHS in England, Wales, Scotland and Northern Ireland, covers the uses of TMS for the purpose of treating and preventing migraine. It sets out recommendations for the use of TMS as treatment during a migraine, or as a possible preventive measure for those who regularly suffer from migraines. It is based on a review of recent studies which found some evidence of beneficial outcomes and no evidence of serious risks associated with having TMS procedures in the short term.
However, NICE emphasises that there is not enough evidence about how safe or effective it is in the long term or when used often, and recommends that it is only used in specialist clinics and with special arrangements for performance review. Along with the guidance, NICE has produced a ‘clinical audit tool’ which includes audit criteria and a data collection form to help clinics review on a regular basis how the procedure is used and its outcomes. It also recommends that any serious patient safety incidents should be reported to the National Reporting and Learning System. Finally, they encourage further research on TMS for prevention and treatment of migraine.
This development is in line with recommendations made by the Council in its 2013 report on novel neurotechnologies that more should be done to bring together and make the most of existing evidence from research and from new treatments, such as TMS.
The Council proposed that this could be achieved by the establishment of publicly accessible registers of information about the uses of such technologies in treatment or research, through the joint efforts of professional bodies such as the Association of British Neurologists, Society of British Neurological Surgeons and the Royal College of Psychiatrists.
Such registers, the Council said, would not only be useful to clinicians and researchers, but also to patients, families and carers in helping them make informed treatment choices. The Council recently held a roundtable meeting with clinicians and other stakeholders to discuss the possibility of setting up a clinical registry for Deep Brain Stimulation, a more invasive form of treatment used in some cases of severe disorders such as Parkinson’s disease.
Find out more about the Council’s work on novel neurotechnologies.
Read a blog post about the Council’s recent workshop on the possibility of creating registries for brain stimulation treatments.

Find out more about how TMS works
The guidance, issued to the NHS in England, Wales, Scotland and Northern Ireland, covers the uses of TMS for the purpose of treating and preventing migraine. It sets out recommendations for the use of TMS as treatment during a migraine, or as a possible preventive measure for those who regularly suffer from migraines. It is based on a review of recent studies which found some evidence of beneficial outcomes and no evidence of serious risks associated with having TMS procedures in the short term.
However, NICE emphasises that there is not enough evidence about how safe or effective it is in the long term or when used often, and recommends that it is only used in specialist clinics and with special arrangements for performance review. Along with the guidance, NICE has produced a ‘clinical audit tool’ which includes audit criteria and a data collection form to help clinics review on a regular basis how the procedure is used and its outcomes. It also recommends that any serious patient safety incidents should be reported to the National Reporting and Learning System. Finally, they encourage further research on TMS for prevention and treatment of migraine.
This development is in line with recommendations made by the Council in its 2013 report on novel neurotechnologies that more should be done to bring together and make the most of existing evidence from research and from new treatments, such as TMS.
The Council proposed that this could be achieved by the establishment of publicly accessible registers of information about the uses of such technologies in treatment or research, through the joint efforts of professional bodies such as the Association of British Neurologists, Society of British Neurological Surgeons and the Royal College of Psychiatrists.
Such registers, the Council said, would not only be useful to clinicians and researchers, but also to patients, families and carers in helping them make informed treatment choices. The Council recently held a roundtable meeting with clinicians and other stakeholders to discuss the possibility of setting up a clinical registry for Deep Brain Stimulation, a more invasive form of treatment used in some cases of severe disorders such as Parkinson’s disease.
Find out more about the Council’s work on novel neurotechnologies.
Read a blog post about the Council’s recent workshop on the possibility of creating registries for brain stimulation treatments.
Share